A molecular movie of interatomic Coulombic decay
|
During the last 15 years a novel decay mechanism of excited atoms has been discovered and investigated. This so-called interatomic Coulombic decay (ICD) involves the chemical environment of the electronically excited atom: the excitation energy is transferred (in many cases over long distances) to a neighbor of the initially excited particle usually ionizing that neighbor. It turned out that ICD is a very common decay route in nature as it occurs across van der Waals and hydrogen bonds. The time evolution of ICD is predicted to be highly complex, as its efficiency strongly depends on the distance of the atoms involved and this distance typically changes during the decay. Here we present the first direct measurement of the temporal evolution of ICD using a novel experimental approach. 
Fig.1: Sketch of the potential energy diagram of the states involved in the process. 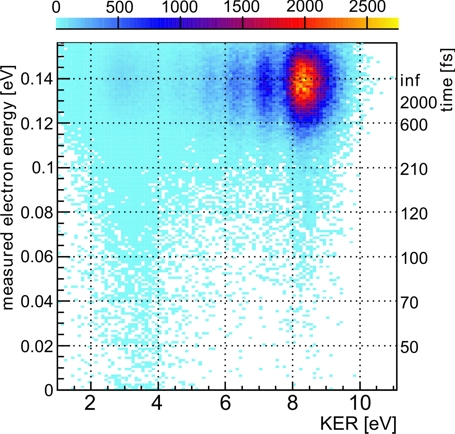
Fig.2: Electron energies and kinetic energy releases measured in coincidence. Each decay time belongs to a certain measured electron kinetic energy. The decay times obtained are shown on the right. 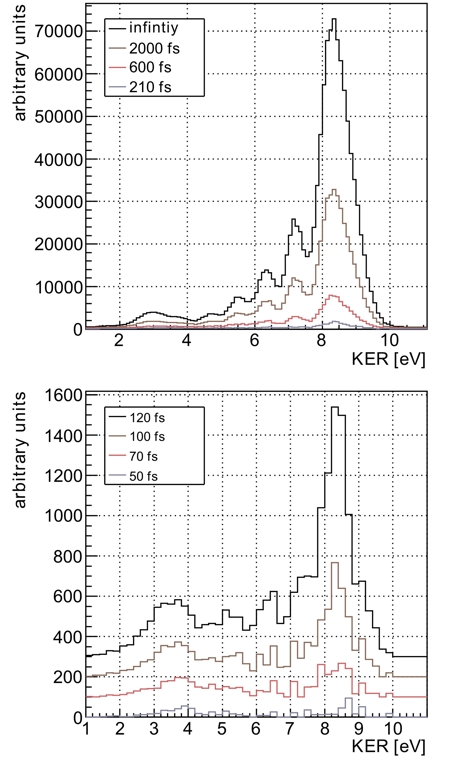
Fig.3: Measured KER distributions for different decay times. The decay is nonexponential as both theory and experiment reveal: for shortest decay times the decay rates are small as typical internuclear distances are large. As the dimer contracts during later decay times, the rates increase and keep showing the nonexponential behavior that is determined by nuclear motion. In conclusion, we have added a new powerful streaking approach to the toolbox of ultrafast science and applied it to visualize the time dependence of an interatomic decay process. The results directly show the evolution of the vibrational wave packet of a helium dimer during the decay and thus give insight into the complex behavior of ICD in the time domain. The measurement approach presented here can be used to investigate other processes and systems in the time domain as well. Experiments investigating the evolution of a hole created inside an atom or molecule could be traced in time in the future using the same approach. Publication:
F. Trinter, J. B. Williams, M. Weller, M. Waitz, M. Pitzer, J. Voigtsberger, C. Schober, G. Kastirke, C. Müller, C. Goihl, P. Burzynski, F. Wiegandt, T. Bauer, R. Wallauer, H. Sann, A. Kalinin, L. Ph. H. Schmidt, M. Schöffler, N. Sisourat, and T. Jahnke |