Resonant Auger decay driving intermolecular Coulombic decay in molecular dimers
|
In 1997, it was predicted that an electronically excited atom or molecule placed in a loosely bound chemical system (such as a hydrogen-bonded or van-der-Waals-bonded cluster) could efficiently decay by transferring its excess energy to a neighbouring species that would then emit a low-energy electron. This intermolecular Coulombic decay (ICD) process has since been shown to be a common phenomenon, raising questions about its role in DNA damage induced by ionizing radiation, in which low-energy electrons are known to play an important part. It was recently suggested that ICD can be triggered efficiently and site-selectively by resonantly core-exciting a target atom, which then transforms through Auger decay into an ionic species with sufficiently high excitation energy to permit ICD to occur. Here we show experimentally that resonant Auger decay can indeed trigger ICD in dimers of both molecular nitrogen and carbon monoxide. By using ion and electron momentum spectroscopy to measure simultaneously the charged species created in the resonant-Auger-driven ICD cascade, we find that ICD occurs in less time than the 20 femtoseconds it would take for individual molecules to undergo dissociation. Our experimental confirmation of this process and its efficiency may trigger renewed efforts to develop resonant X-ray excitation schemes for more localized and targeted cancer radiation therapy. 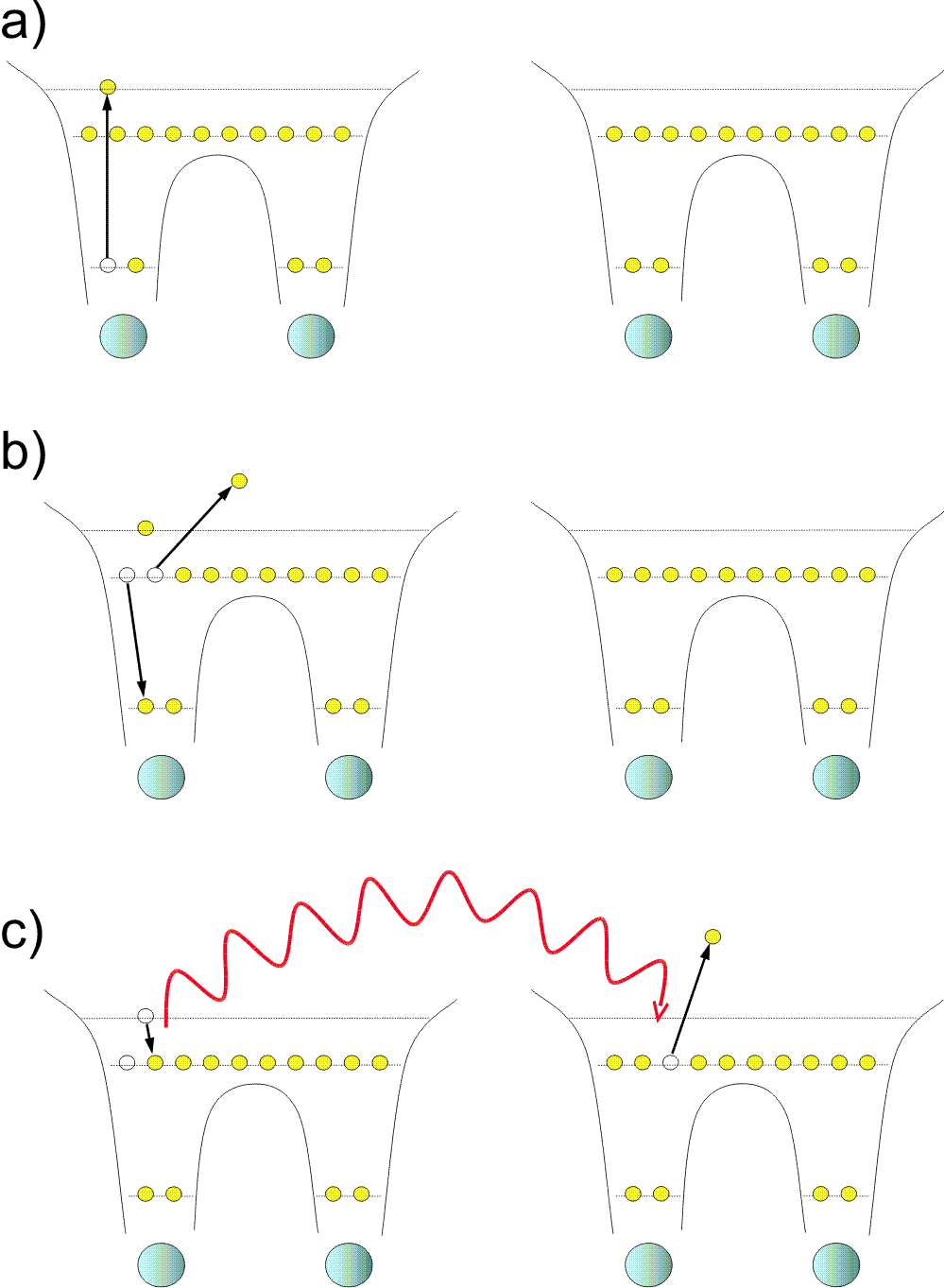
Fig. 1: The overall decay cascade mechanism: Shown is the series of events involved in resonant-Auger-driven ICD. a) One molecule (left) of the molecular dimer is core-excited. b) The core-excited state decays by a spectator Auger decay to a highly excited state of the molecular ion. c) ICD transfers the excitation energy to the molecular neighbour (right), where a low-energy ICD electron is emitted. 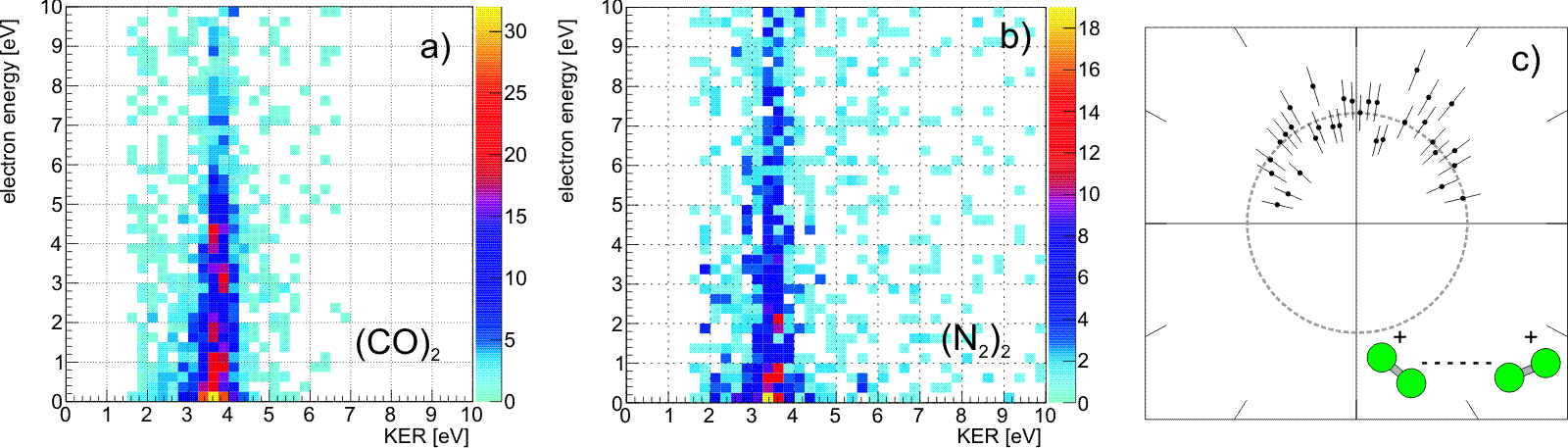
Fig. 2: Experimental results: a) Kinetic energy release of (CO)2 versus energy of one of the two electrons created by ICD after resonant excitation and subsequent Auger decay at a photon energy of 287.4 eV (Π* excitation of CO). The colour scale shows the intensity in counts. b) Same plot for (N2)2 recorded at a photon energy of 401.9 eV (Π* excitation of N2). c) Emission direction of the Auger electron with respect to the molecular axis of the N2 dimer (with statistical error bars). The dimer is oriented horizontally, as depicted by the green icon. The grey circle is a line to guide the eye, corresponding to isotropic emission. Publication:
F. Trinter, M. S. Schöffler, H.-K. Kim, F. P. Sturm, K. Cole, N. Neumann, A. Vredenborg, J. Williams, I. Bocharova, R. Guillemin, M. Simon, A. Belkacem, A. L. Landers, Th. Weber, H. Schmidt-Böcking, R. Dörner & T. Jahnke Press releases:
Regiomelder Frankfurt (german press release) Collaborators:
Lawrence Berkeley National Laboratory, Chemical Sciences Division, Berkeley, California 94720, USA |